For topics which don't fit anywhere else! Discuss the weather, your mood, hobbies and interests. Remember, keep it child-friendly

 by CᴀɴᴅʏNᴜᴛᴍᴇɢ » Tue Sep 03, 2019 3:57 pm
by CᴀɴᴅʏNᴜᴛᴍᴇɢ » Tue Sep 03, 2019 3:57 pm
long shoe wrote:I HAVE A QUESTION!
TYPE OF QUESTION:chemistry
YOUR QUESTION:okay so this is kinda a lot but any help on any question would be VERY appreciated! im completely clueless. i’m a junior taking AP biology, i’ve never taken chemistry in my life so i’m completely clueless. this is all of the “essential” chemistry for ap bio.
Ooh, stoichiometry! I'd have to review the biochem and functional groups, that's not my forte, but I can explain parts of this.
Earlier on the page you said that molarity is moles of solute over liters (of solution). Great! Write that on your paper a few more times, if it helps, since that's the key for the next few questions.
19.C) 1. 10.0g of sulphuric acid + 0.1L of water, find the molarity.
Here, you're given grams of H2SO4. Since that's the solute (the compound being dissolved), and you need to know the quantity of moles of the solute to be able to find molarity, find the equivalent of 10g in moles.
To do this, divide the mass (10g) by the molar mass of H2SO4. (Why? Check with a unit conversion; you're trying to find moles. (g)/(g/mol) = (g)(mol/g), grams cancel out = moles.)
Molar mass of H2SO4 = the combined masses of all its components = (2 x molar mass of hydrogen) + (1 x molar mass of sulphur) + (4 x molar mass of oxygen).
Google tells me it's 98.08g/mol, but make sure you know where on the periodic table you can find these masses during your tests, and check that you are able to calculate the correct final mass several times.
Okay, 10g/98.08g/mol = 0.102mol of H2SO4.
Molarity = moles of solute / litres of solution, and you've got both now.
0.102mol H2SO4 / 0.1L H2O = 1.02M.
(Note, capital M is the symbol for molarity - I think that was another question for you.)
Does this make sense? The other questions under 19C are either very similar, or the same with the reverse process.
Try out another one, feel free to let me know if you have questions. c:
--
15.
Try this link - https://science.howstuffworks.com/envir ... s/h2o7.htm, it has pretty much all the terms you're looking for.
Cohesion and adhesion - if you're in bio, the example you'll see at some point is water travelling up plant xylem. Cohesion is the water particles sticking to each other, adhesion is the particles sticking to other particles. Therefore, in the plant stem, the water adheres to the inside of the stem, and also coheres to itself. (As an aside, this is the first time I have ever used the verb 'to cohere' - I had to search up whether it exists, but it does!)
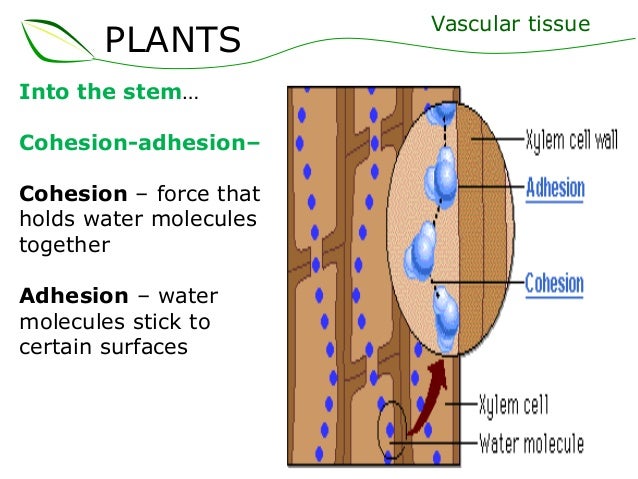
--
16. Water is unique in that the solid state is less dense than the liquid state. This happens because ice is organized into a lattice while water is relatively mobile:

Try to search up the why if you're interested in looking at this further; I suggest this link, https://chemistry.stackexchange.com/que ... than-water, but it may be too technical.
--
Please do more research into these! It's almost midnight here and I'm just giving you a place to start, since you said you couldn't find anything specific online.
17b. "What is it about water molecules and the ions in salt that might make water able to dissolve salt?
The positive and negative polar ends of a water molecule are attracted to the negative chloride ions and positive sodium ions in the salt." from https://www.middleschoolchemistry.com/l ... r5/lesson3.
The polar ends pull NaCl (and other ionic compounds) apart. For larger molecules like sucrose, try here: https://chemed.chem.purdue.edu/genchem/ ... oluble.php
c. https://www.usgs.gov/special-topic/wate ... er_objects
d. Basically, sweat is primarily water (with some salts and other components), and water has a high specific heat capacity (requires a lot of energy/heat to heat up). So, your body exudes this water onto the skin, heats it up (therefore using up body heat), the water in the sweat evaporates and takes the heat it used to do so with it.
e, f. http://www.appstate.edu/~goodmanjm/rcoe ... waterdrops
g. Same concept as water moving up a plant stem. https://www.usgs.gov/special-topic/wate ... er_objects
Last edited by
CᴀɴᴅʏNᴜᴛᴍᴇɢ on Mon Dec 23, 2019 8:38 pm, edited 1 time in total.
-

CᴀɴᴅʏNᴜᴛᴍᴇɢ
-
- Posts: 802
- Joined: Mon Nov 12, 2012 1:10 pm
- My pets
- My items
- My wishlist
- My gallery
- My scenes
- My dressups
- Trade with me
 by bellamare » Wed Sep 04, 2019 1:11 am
by bellamare » Wed Sep 04, 2019 1:11 am
CᴀɴᴅʏNᴜᴛᴍᴇɢ wrote:long shoe wrote:I HAVE A QUESTION!
TYPE OF QUESTION:chemistry
YOUR QUESTION:okay so this is kinda a lot but any help on any question would be VERY appreciated! im completely clueless. i’m a junior taking AP biology, i’ve never taken chemistry in my life so i’m completely clueless. this is all of the “essential” chemistry for ap bio.
Ooh, stoichiometry! I'd have to review the biochem and functional groups, that's not my forte, but I can explain parts of this.
Earlier on the page you said that molarity is moles of solute over liters (of solution). Great! Write that on your paper a few more times, if it helps, since that's the key for the next few questions.
19.C) 1. 10.0g of sulphuric acid + 0.1L of water, find the molarity.
Here, you're given grams of H2SO4. Since that's the solute (the compound being dissolved), and you need to know the quantity of moles of the solute to be able to find molarity, find the equivalent of 10g in moles.
To do this, divide the mass (10g) by the molar mass of H2SO4. (Why? Check with a unit conversion; you're trying to find moles. (g)/(g/mol) = (g)(mol/g), grams cancel out = moles.)
Molar mass of H2SO4 = the combined masses of all its components = (2 x molar mass of hydrogen) + (1 x molar mass of sulphur) + (4 x molar mass of oxygen).
Google tells me it's 98.08g/mol, but make sure you know where on the periodic table you can find these masses during your tests, and check that you are able to calculate the correct final mass several times.
Okay, 10g/98.08g/mol = 0.102mol of H2SO4.
Molarity = moles of solute / litres of solution, and you've got both now.
0.102mol H2SO4 / 0.1L H2O = 1.02M.
(Note, capital M is the symbol for molarity - I think that was another question for you.)
Does this make sense? The other questions under 19C are either very similar, or the same with the reverse process.
Try out another one, feel free to let me know if you have questions. c:
--
15.
Try this link - https://science.howstuffworks.com/envir ... s/h2o7.htm, it has pretty much all the terms you're looking for.
Cohesion and adhesion - if you're in bio, the example you'll see at some point is water travelling up plant xylem. Cohesion is the water particles sticking to each other, adhesion is the particles sticking to other particles. Therefore, in the plant stem, the water adheres to the inside of the stem, and also coheres to itself. (As an aside, this is the first time I have ever used the verb 'to cohere' - I had to search up whether it exists, but it does!)

--
16. Water is unique in that the solid state is less dense than the liquid state. This happens because ice is organized into a lattice while water is relatively mobile:

Try to search up the why if you're interested in looking at this further; I suggest this link, https://chemistry.stackexchange.com/que ... than-water, but it may be too technical.
--
Please do more research into these! It's almost midnight here and I'm just giving you a place to start, since you said you couldn't find anything specific online.
17b. "What is it about water molecules and the ions in salt that might make water able to dissolve salt?
The positive and negative polar ends of a water molecule are attracted to the negative chloride ions and positive sodium ions in the salt." from https://www.middleschoolchemistry.com/l ... r5/lesson3.
The polar ends pull NaCl (and other ionic compounds) apart. For larger molecules like surcose, try here: https://chemed.chem.purdue.edu/genchem/ ... oluble.php
c. https://www.usgs.gov/special-topic/wate ... er_objects
d. Basically, sweat is primarily water (with some salts and other components), and water has a high specific heat capacity (requires a lot of energy/heat to heat up). So, your body exudes this water onto the skin, heats it up (therefore using up body heat), the water in the sweat evaporates and takes the heat it used to do so with it.
e, f. http://www.appstate.edu/~goodmanjm/rcoe ... waterdrops
g. Same concept as water moving up a plant stem. https://www.usgs.gov/special-topic/wate ... er_objects
omg thank you so much this is so helpful!! i didn’t exoect to get an answer this in depth and so much! thank you for relating to biology and for all the sources to read up on, i definitely will. i appreciate this more than i can express, again, thank you so so much 💕💕💕💕
bellamarecontact mediscord: bellamare#3663
art creditpfp: me
signature:
softsweetmello
adult - it/she - pansexual - canine therian
part time student, vet tech and furry artist
links
lf art
art shop
ocs ufs/uft
-

bellamare
-
- Posts: 7742
- Joined: Wed Jul 01, 2015 3:24 am
- My pets
- My items
- My wishlist
- My gallery
- My scenes
- My dressups
- Trade with me
-
 by CᴀɴᴅʏNᴜᴛᴍᴇɢ » Sat Sep 07, 2019 3:32 pm
by CᴀɴᴅʏNᴜᴛᴍᴇɢ » Sat Sep 07, 2019 3:32 pm
Blumenkranz wrote:I HAVE A QUESTION!
TYPE OF QUESTION: Trigonometry
YOUR QUESTION: Show that cos(-45°) = cos45°
My teacher didn't give any examples so I'm not sure of what to do? Thank you!

(from the top answer in https://math.stackexchange.com/question ... s-negative - check out the link for more explanation!)
In the unit circle, cos is the x coordinate. Therefore, rotating counterclockwise (positive) and clockwise (negative) from the x-axis for an equal angle produces an equal x value.
You could also prove this by sketching the function and indicating that it is even therefore equal x-distances on either side of the y-axis would give equal y-values (cos values).
-

CᴀɴᴅʏNᴜᴛᴍᴇɢ
-
- Posts: 802
- Joined: Mon Nov 12, 2012 1:10 pm
- My pets
- My items
- My wishlist
- My gallery
- My scenes
- My dressups
- Trade with me
 by hannur » Mon Sep 09, 2019 9:32 pm
by hannur » Mon Sep 09, 2019 9:32 pm
I HAVE A QUESTION!
TYPE OF QUESTION: ermm, lingustics?
YOUR QUESTION: would there be any explanation for how words like 'quilt' are different to words such as 'cat'?
for context i replicated an experiment involving getting participants to recall words from a word list, and i observed a Von Restorff effect in the word 'quilt', where every single participant somehow managed to recall this word... i just want a way to justify why this may have been the case, when the other words in my word list were words like book, cat, ring, pen etc etc
thank you so much
-

hannur
-
- Posts: 1169
- Joined: Sat May 13, 2017 3:07 pm
- My pets
- My items
- My wishlist
- My gallery
- My scenes
- My dressups
- Trade with me
 by Schuyler » Mon Sep 09, 2019 10:06 pm
by Schuyler » Mon Sep 09, 2019 10:06 pm
seafoamcastle wrote:I HAVE A QUESTION!
TYPE OF QUESTION: ermm, lingustics?
YOUR QUESTION: would there be any explanation for how words like 'quilt' are different to words such as 'cat'?
for context i replicated an experiment involving getting participants to recall words from a word list, and i observed a Von Restorff effect in the word 'quilt', where every single participant somehow managed to recall this word... i just want a way to justify why this may have been the case, when the other words in my word list were words like book, cat, ring, pen etc etc
thank you so much
Hmm, that's interesting. So the experiment was that the participants were given a list of words and then asked to recall what the words were from memory, is that right? From a linguistic perspective, I can't think of anything particular about "quilt" that's different from other nouns, but my thought is that it stood out more to the participants and therefore was easier to recall later simply because it doesn't come up nearly as often in the typical everyday conversation as the other words you listed, so it registered as "unusual" while the others were just "normal" words.


 .
.


 Names: Schuyler, Schuye, or Nárë/Narwë
Names: Schuyler, Schuye, or Nárë/Narwë
-

Schuyler
- General Helper
-
- Posts: 8713
- Joined: Mon Jun 06, 2011 6:56 am
- My pets
- My items
- My wishlist
- My gallery
- My scenes
- My dressups
- Trade with me
-
 by chooch » Tue Sep 10, 2019 9:40 am
by chooch » Tue Sep 10, 2019 9:40 am
I HAVE A QUESTION!
TYPE OF QUESTION: algebra 2: linear systems
YOUR QUESTION:
2x + 5y = 8
4x = 16 - 10y
i turned it into this:
2x + 5y = 8
4x + 10y = 16
we have to use the elimination method for this question, but i'm not sure how to continue. i'd appreciate any help. thank you!
■ she/her, leo, isfp-t
| about
-

chooch
-
- Posts: 3257
- Joined: Mon Apr 13, 2015 11:15 am
- My pets
- My items
- My wishlist
- My gallery
- My scenes
- My dressups
- Trade with me
 by .Cas. » Tue Sep 10, 2019 9:43 am
by .Cas. » Tue Sep 10, 2019 9:43 am
pokus wrote:I HAVE A QUESTION!
TYPE OF QUESTION: algebra 2: linear systems
YOUR QUESTION:
2x + 5y = 8
4x = 16 - 10y
i turned it into this:
2x + 5y = 8
4x + 10y = 16
we have to use the elimination method for this question, but i'm not sure how to continue. i'd appreciate any help. thank you!
Well, I’d suggest multiplying the top by negative two. That will give -4x-10y= -16
It looks like everything cancels, actually. I think that would just make your answer 0?
I’m leaving the site. Anything that is unlocked in my trades or items is up for grabs. PM me for locked pets or C$
-
.Cas.
-
- Posts: 2782
- Joined: Mon Jan 01, 2018 11:20 am
- My pets
- My items
- My wishlist
- My gallery
- My scenes
- My dressups
- Trade with me
Who is online
Users browsing this forum: No registered users and 15 guests















































